zinc valence electrons|periodic table with valence electrons : Bacolod Mar 23, 2023 The best EGT crypto games are determined by the preferences of Bitcoin casino players. These EGT slots online games stand out among casino visitors: Gold Dust - Offers the potential for significant winnings, with a maximum payout of 50,000 times the bet.;Circus Brilliant - A story-driven video slot with the potential for up to 10,000 times the bet in .
PH0 · valence orbital diagram zn
PH1 · valence electrons chart
PH2 · periodic table with valence electrons
PH3 · orbital diagram for zn
PH4 · list of valence electrons for each element
PH5 · how to calculate valence electrons
PH6 · how many electrons does zinc have
PH7 · electron configuration of zn
PH8 · Iba pa
Watch Bisaya Pinay Scandal porn videos for free, here on Pornhub.com. Discover the growing collection of high quality Most Relevant XXX movies and clips. No other sex tube is more popular and features more Bisaya Pinay Scandal scenes than Pornhub! Browse through our impressive selection of porn videos in HD quality on any device you own.
zinc valence electrons*******Mar 23, 2023
Zinc has a valence of +2, meaning it can form compounds with two .
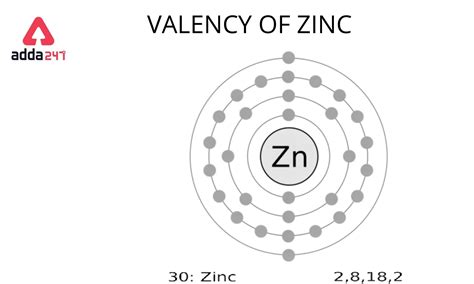
To find the number of valence electrons for Zinc (Zn) we need to look at its electron configuration. This is necessary because Zn is a transition metal (d block element) and we need to take.
zinc valence electrons To find the number of valence electrons for Zinc (Zn) we need to look at its electron configuration. This is necessary because Zn is a transition metal (d block element) and we need to take.zinc valence electrons periodic table with valence electrons To find the number of valence electrons for Zinc (Zn) we need to look at its electron configuration. This is necessary because Zn is a transition metal (d block element) and we need to take. Zinc has only 2 valence electrons, both located in the 4s-orbital. This is based on its electron configuration and its ability to lose these electrons to form Zn2+ cation. See the detailed answer and . Zn – 2e – → Zn 2+. Here, the electron configuration of zinc ion is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. This electron configuration shows that zinc ion (Zn 2+) has three shells and the last shell has .Learn how to determine the number of valence electrons for an element using the periodic table. Zinc is in group 12 and has two valence electrons, except for zincate ions . Get to know everything about the Zinc valence electrons here in the article. We shall break out the key information of the element for readers. Zinc is a well . Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2 s subshell and four in the 2 p subshell. We can write the configuration of oxygen's valence . Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical . Valence electrons are the outer-shell electrons of an atom. Valence electrons determine the reactivity of an atom. Atoms have a tendency to have eight . In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. Valence electrons are outer shell electrons for .An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can .

That is, there are thirty electrons in the atom of the zinc element. So, it is possible to determine the properties of zinc from the electron configuration. Now, the electron configuration of zinc shows .
Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd . 3.1: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..The Valence Orbital Diagram for Zinc (Zn) Zinc (Zn) is a transition metal that has a valence electron configuration of [Ar] 3d 10 4s 2. This means that in its ground state, zinc has two valence electrons. The valence orbital diagram is a way to represent these electrons in their respective energy levels and orbitals.
The valence electrons are the electrons that determine the most typical bonding patterns for an element. These electrons are found in the s and p orbitals of the highest energy level for the element. Sodium 1s22s22p63s1. Sodium has 1 valence electron from the 3s orbital. Phosphorus 1s22s22p63s23p3. Phosphorus has 5 valence electrons 2 from the .Zinc is a chemical element of the periodic table with chemical symbol Zn and atomic number 30 with an atomic weight of 65.382 u and is classed as a transition metal. . Valence electrons : 2: Valency electrons : 2: Bohr model: Electron shell for Zinc, created by Injosoft AB Zn. Figure: Shell diagram of Zinc (Zn) atom. Orbital Diagram. 1s: 2s .
Call Our Hotline +632 8877-7222. For Domestic Toll Free 1-800-10000-7222. For RCBC Credit Cards +632 8888-1888. Yuchengco Tower RCBC Plaza 6819 Ayala Avenue
zinc valence electrons|periodic table with valence electrons